Thalidomide: The Tragedy Of Distaval & Birth Defects - What You Need To Know
Could a seemingly harmless medication, once touted as a safe remedy, unleash a wave of unimaginable suffering? The story of thalidomide, a drug prescribed to pregnant women, remains a chilling testament to the devastating consequences of unchecked pharmaceutical practices and the importance of rigorous safety protocols.
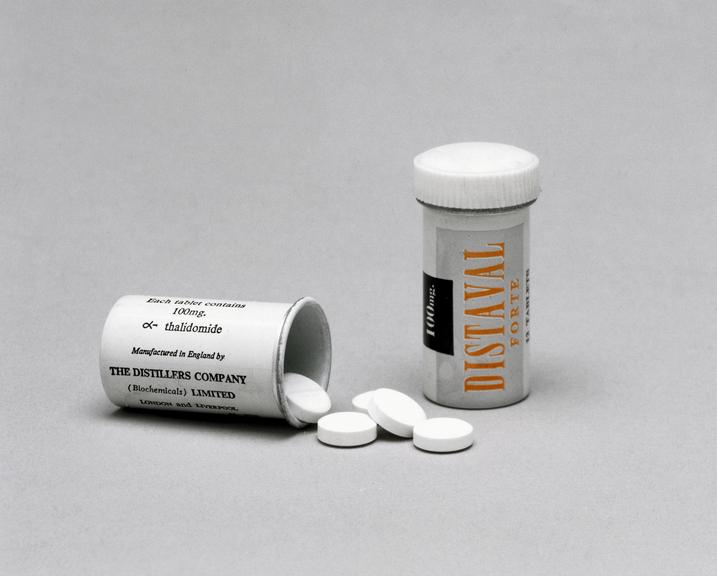
In the annals of medical history, few tragedies cast as long a shadow as the thalidomide disaster. Developed in Germany in 1954 by Chemie Grnenthal, thalidomide initially promised relief from morning sickness, anxiety, and insomnia. Marketed under various brand names, including Distaval in the United Kingdom and Contergan in Germany, the drug quickly gained popularity across Europe, Australia, and even parts of Canada. Its supposed safety, even for pregnant women, made it a seemingly ideal solution for a range of ailments.
However, this widespread use would soon lead to one of the biggest anthropogenic medical disasters ever witnessed. The sedative, initially marketed as a safe and effective remedy, was unknowingly causing severe birth defects in thousands of children. The full extent of the tragedy unfolded gradually, as doctors began to notice a disturbing pattern: an alarming rise in babies born with phocomelia a condition characterized by malformed or absent limbs. The link between thalidomide and these devastating deformities became increasingly undeniable.
| Category | Details |
|---|---|
| Drug Name | Thalidomide |
| Original Developer | Chemie Grnenthal (Germany) |
| Year of Development | 1954 |
| Marketed Under (UK) | Distaval (Distillers (Biochemicals) Ltd) |
| Primary Use | Relief from morning sickness, anxiety, and insomnia |
| Countries Affected | 46 countries |
| Years of Use | Late 1950s - Early 1960s |
| Known Effects | Phocomelia (malformed or absent limbs) and other severe deformities |
| Number of Affected Children | Over 10,000 with severe deformities |
| Withdrawal | Early 1960s |
| Mechanism of Action (Birth Defects) | Inhibition of cell growth in developing limbs |
| Current use | Used in treatment of Erythema Nodosum leprosum (ENL) |
The drug's introduction in the United Kingdom in 1958, under the brand name Distaval, marked the beginning of a period of widespread prescription. Marketed by Distillers (Biochemicals) Ltd, the drug was promoted as a safe remedy for morning sickness and was readily available through both the National Health Service (NHS) and private doctors. Advertisements assured pregnant women and nursing mothers of its complete safety, promising no adverse effects on either mother or child. However, this seemingly benign medication was insidiously harming the very population it was meant to help.
The widespread distribution of thalidomide, in over 46 countries, painted a grim picture of a pharmaceutical tragedy on an international scale. While it was most frequently associated with morning sickness, the drug was also prescribed for a range of other conditions, including pneumonia, colds, flu, and the symptoms of headache, toothache, hay fever, bronchitis, migraine, fibrositis, asthma, neuralgia, and arthritis. The potential for such broad application, combined with the misleading perception of safety, meant that countless women were exposed to the drug during the critical stages of pregnancy.
The discovery of the link between thalidomide and birth defects was not immediate. In the early stages, many doctors dismissed the growing concerns as coincidences. However, as more and more cases of limb deformities emerged, a growing number of medical professionals began to suspect a correlation. The sheer prevalence of affected children, coupled with the fact that these deformities were often unique, ultimately pointed to an environmental cause. This led to investigations into potential factors and, eventually, to the inevitable recognition that thalidomide was the culprit.
Among the key figures who challenged the initial assurances of safety was Dr. William McBride, an Australian obstetrician. Observing a surge in birth defects, McBride suspected a connection to a medication, specifically Distaval. He voiced his concerns, writing to the manufacturer and urging them to withdraw the drug. His efforts were met with resistance, highlighting the powerful influence of pharmaceutical companies and the early reluctance to acknowledge potential risks. Despite facing skepticism, McBride's persistent advocacy played a crucial role in raising awareness.
Another doctor, Dr. Widukind Lenz in Germany, also played a vital role in uncovering the truth. Lenz meticulously documented cases and analyzed the pattern of birth defects, ultimately providing compelling evidence linking thalidomide to the malformations. His work and the persistence of others helped to convince authorities to take action, but only after a significant toll had been taken. The delay in recognizing the dangers cost countless children their normal lives, and the repercussions of the thalidomide disaster are still felt today.
The withdrawal of thalidomide, in the early 1960s, was a turning point. In the UK, the drug was pulled from the market by November 1961. This act, though late, marked a significant step towards acknowledging the devastation and preventing further harm. The immediate aftermath saw a scramble for answers, investigations into the drug's effects, and a growing public outcry about the negligence of the pharmaceutical companies involved.
The scale of the thalidomide tragedy is staggering. The most tragic aspect is the sheer number of children affected. It is estimated that over 10,000 children worldwide were born with severe deformities directly linked to the drug. These deformities included shortened or absent limbs, but other serious issues also affected their eyes, ears, hearts, and internal organs. The psychological impact on the families, who faced the realities of raising disabled children, was immeasurable.
The thalidomide disaster exposed serious flaws in the drug approval and regulation processes of the time. Before the tragedy, there was a significant lack of stringent testing requirements, especially regarding the effects of drugs on pregnant women. It became painfully clear that the scientific community and regulatory bodies had fallen short in protecting the public from pharmaceutical risks. The disaster has led to significant reforms in drug safety regulations worldwide. The need for thorough testing, especially on pregnant animals, was realized, along with the importance of post-market surveillance to monitor drugs for any unexpected adverse effects.
The scientific understanding of how thalidomide causes birth defects continues to evolve. While the exact mechanism is still under research, it is believed that the drug inhibits the formation of blood vessels, disrupting normal cell growth in developing limbs. It is also thought to interfere with the production of certain proteins essential for fetal development. However, the precise biochemical pathways affected by thalidomide are still a subject of ongoing scientific inquiry.
In a cruel twist of fate, thalidomide has, in recent years, found new uses in the medical field. It has been shown to be effective in treating certain cancers, such as multiple myeloma, and is also used in the treatment of erythema nodosum leprosum (ENL), a painful complication of leprosy. While these applications offer a degree of redemption, they also serve as a stark reminder of the complex nature of medicine and the lasting impact of the thalidomide tragedy.
Today, thalidomide is only available under strict regulations, with careful monitoring of patients. It is an example of the ongoing need for vigilance in the pharmaceutical industry. It serves as a reminder of the importance of protecting vulnerable populations. The story of thalidomide is a cautionary tale of the dangers of unchecked practices, underscoring the necessity of rigorous drug testing, rigorous oversight, and a commitment to putting patient safety above all else.
The thalidomide tragedy is not only about the physical deformities of the affected children. It is also a story of corporate negligence, regulatory failures, and the enduring power of advocacy. Its a call for accountability and transparency in the pharmaceutical industry. The lessons learned from the disaster continue to shape modern medicine and are a testament to the importance of vigilance and the ongoing pursuit of patient safety.